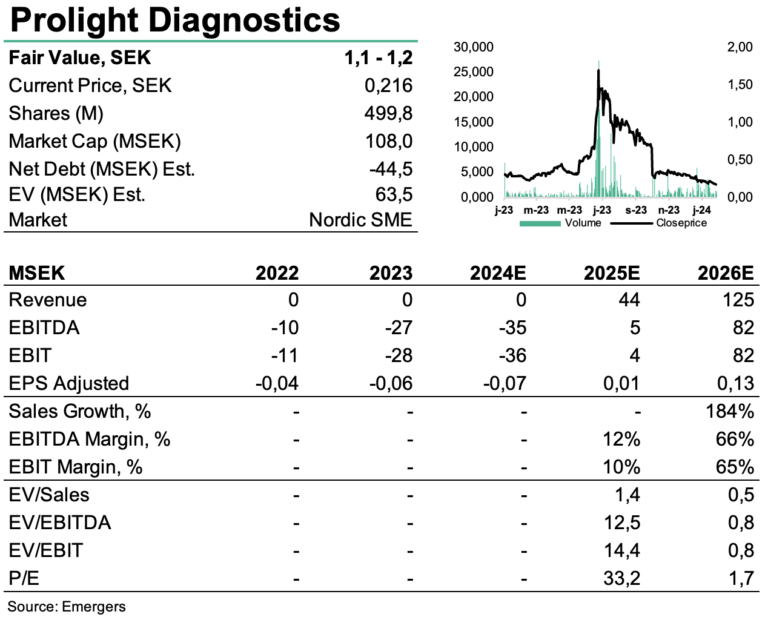
PROLIGHT: PoC troponin testing ready for next step

With proof-of-performance for its proprietary digital technique for detection of high-sensitive biomarkers even in whole blood, Prolight has taken significant steps forward in the past year. The share however, has experienced extended downwards pressure following the unit issue and subsequent compensation issue for the underwriters. Once the underwriters’ 150m shares have found their way to more sturdy investors, and the company continues to make progress with prototype development and IVDR certification towards launch in 2026, we expect the pressure to ease, and a revaluation that better reflects the long term prospects, where we find support for a fair value of SEK 1.1 – 1.2 per share.
No surprises in Q4
Just like same period last year, Prolight had no sales in Q4’23. Other operating income, mainly related to tax-related grants in Psyros for research and development, amounted to SEK 5.9m. OPEX in Q4’23 amounted to SEK 9.9m. Total cash flow in Q4 amounted to SEK -4m, leaving net cash at the end of 2023 at SEK 13.3m. This did not however include the Paid Subscription Units (”BTU”) subscribed to in the company’s rights issue that was carried out in December 2023. With the proceeds from the raise, cash was strengthened by another SEK 31.2m.
Significant long term potential
Prolight has developed a single molecule counting (digital) immuno analysis device that enables detection of biomarkers at extremely low concentrations, using a single drop of blood. Diagnostic methods available today are associated with long waiting times, whereas Prolight’s device provide results in 10 minutes, at the Point of Care, thus reducing financial burden and patient suffering. Prolight has significant growth potential not only in the USD 1.5 billion troponin testing market but also in other valuable biomarkers such as B-type natriuretic peptide (BNP) for suspected heart failure and D-Dimer for suspected blood clot formation, a market valued at USD 1.2 billion.
Selling pressure likely to ease after underwriters have left
Following the proof-of-performance announced in mid-June, followed by whole blood in November 2023, Prolight now focuses on the development of the commercial instrument prototype for digital immune analysis and the preparations for the IVDR certification, with commercialization of the troponin test expected in 2026. Adding the potential for BNP and D-Dimer POC-tests, this translates to a NPV of SEK 680m, supporting a fair value of SEK 1.1-1.2 per share, factoring in additional new equity of a total of around SEK 100m.
The share has however continued to experience significant selling pressure, which we attribute to the underwriters that received 147.9m in the unit issue and another 2.6m shares in the compensation issue. Once those shares have found their way to more sturdy long-term investors, and the company continues to make progress with prototype development and IVDR certification, we expect the selling pressure to ease and give way to a revaluation that better reflects the company’s long term prospects.