PROLIGHT: Pre-validation readout in Q4 sets stage for multicenter trial in 2025

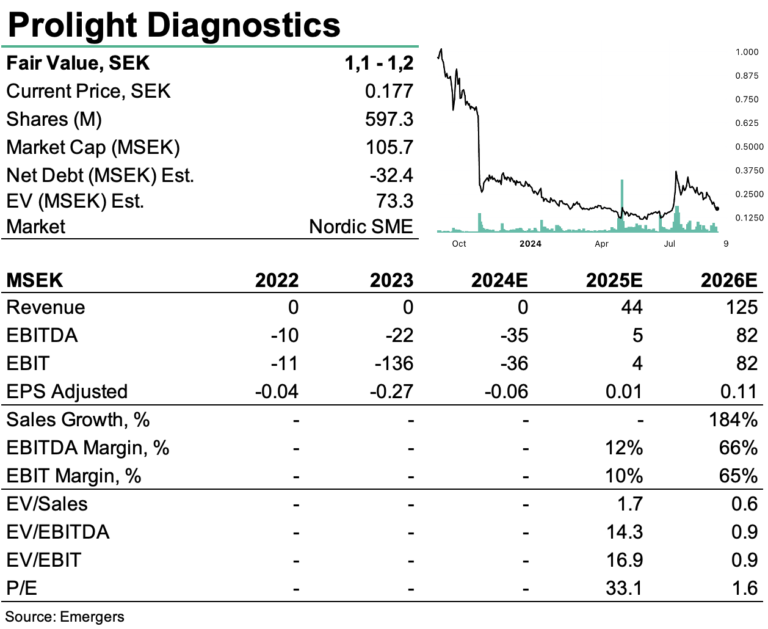
Supported by the latest troponin data generated from whole blood samples on the final commercial cartridge design, Prolight is now ready to initiate trials with banked patient samples and fresh blood samples from patients with suspected heart attack. The first readout is expected in Q4’24, in preparation for the clinical performance study in early 2025. This should further boost interest in Prolight’s solution. With proof-of-performance for the first generation of its proprietary digital technique, focused on the rapid rule-in or rule-out of myocardial infarction by quantifying individual molecules of the protein troponin, the company is on track for IVDR certification and a 2026 launch. Additionally, the potential for future verticals, such as BNP and D-Dimer POC tests, supports a fair value of SEK 1.1-1.2 per share. While the TO6 warrants were only exercised to 45%, raising SEK 9.8m in May, the prestigious SEK 17m NIHR Innovation grant from the UK government provides welcome liquidity and runway.
Advancing prototype in preparation for 2025 multicenter trial
During Q2, Prolight completed the final components for beta prototypes of its instrument, with testing and regulatory verification scheduled for autumn. In collaboration with Flex Medical Solutions, Prolight has finalized a simplified cartridge design for commercial use, which enables cost-effective large-scale manufacturing. The cartridge, containing all necessary reagents, eliminates the need for expensive liquid reagents and blister packs. Its multiplex capability allows multiple biomarkers to be measured from a single drop of blood. Prolight is set to begin a multicenter trial in early 2025.
SEK 9.8m warrant on top of SEK 17m NIHR Innovation grant
During Q2, warrants of series TO6 were exercised to approximately 44.8 percent, at SEK 0.1 per share, raising SEK 9.8m to Prolight. Both the board and management exercised their options in full, and the company reports an increase in the total shareholding of the board and management during H1’24. As reported in conjunction with the Q1 report, Prolight was awarded a SEK 17m grant from the UK’s National Institute of Health and Care Research (NIHR), which will be used for the final steps of the development of the Psyros platform, an encouraging external validation of Prolight’s proprietary Psyros digital point of care system.
Commercialization expected in 2026
PR and marketing efforts at conferences have resulted in more meetings with leading global diagnostic companies at important congresses. Now Prolight is fully focused on the development of the commercial instrument prototype for digital immune analysis and the preparations for the IVDR certification, with commercialization of the troponin test expected in 2026. Adding the potential for BNP and D-Dimer POC-tests, this translates to a NPV of SEK 680m, supporting a fair value of SEK 1.1-1.2 per share, factoring in additional new equity of a total of around SEK 100m. We expect the pre-validation readout in Q4 and multicenter trial in 2025 to be important catalysts to move the share in that direction.