Nanexa AB: NEX-22 Progress, Revaluation and Financing

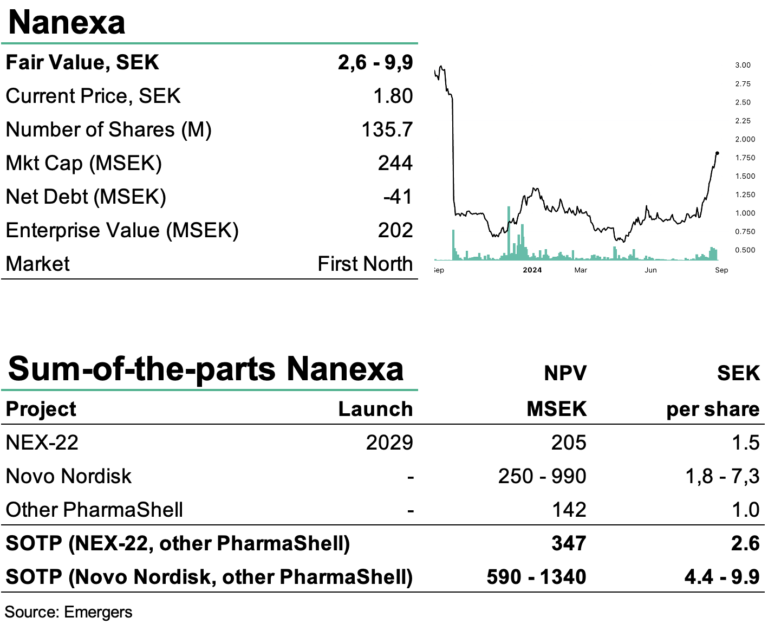
After initiating dosing of the first patient in June with Nanexa’s long-acting depot formulation of the GLP-1 analog liraglutide, NEX-22 for the treatment of type 2 diabetes, phase I is now progressing with the second cohort, according to plan. We now look forward to the outcome from subsequent cohorts and the full Phase I in 2024, and Ib in late 2025, as well as the partner project with Novo Nordisk and other advanced evaluation projects. Since the dip following the CMD in May, the stock has undergone a remarkable revaluation and tripled in value. We continue to find support for a fair value of SEK 2.6-9.9 per share, not factoring in a rights issue, which is the likely scenario if a license deal fails to materialize within the next 6-9 months.
More news on progress with NEX-22 in H2’24
After confirming good pharmacokinetics and tolerability at the injection site, the Phase I study for NEX-22 has now progressed to dose escalation. This confirms that Nanexa is able to handle skin reactions at the injection site (without needing to employ the combination with an anti-inflammatory drug as detailed at the CMD). The timeline for NEX-22 is to complete Phase 1b and have a Pre-IND meeting with the FDA by the end of 2025, in the best-case scenario. Here it is worth keeping in mind that the 505b-path (for new or modified versions of previously approved drugs) to approval can roughly be compared to Phase III for a New Chemical Entity.
For NEX-22, we anticipate that a successful Phase I and Ib/II trial would pave the way for out-licensing opportunities, potentially unlocking a total deal value estimated between USD 300 million (Xplico estimate, with the majority coming from upfront payments and milestones) and USD 800 million (the high end of our estimate, where royalties would constitute a larger part). This could justify an upfront payment of USD 40 million, assuming a 5% peak market share. While NEX-22 is the most interesting and promising opportunity in Nanexa, it is of course closely interlinked with the partner project with Novo Nordisk (that is also Nanexa’s largest shareholder at just shy of 20% of shares). That project is ongoing and scheduled to be concluded in 2025.
License deal needed in 6-9 months to avoid rights issue
Additionally, partner project portfolio consists of monoclonal antibody projects (around half), peptides and small molecule projects. A review of the partner projects at the CMD showed that PharmaShell, in the finalized projects, have produced successful results, but that the projects were either under review or had been deprioritized for reasons outside Nanexa’s control.
With a moderate cash draw during Q2’24 of SEK 6.5m, we now expect expenses to increase as phase I with NEX-22 progresses. Cash at the end of Q2’24 amounted to SEK 41m and Nanexa now has less than 12 months of runway. Based on our SOTP for NEX-22, the Novo Nordisk project and the PharmaShell evaluation deals, we continue to find support for an rNPV of SEK 2.6-9.9 per share. This wide range reflects the wide range of potential outcomes for the company’s various projects and partnerships. But this does not factor in a rights issue, which is the likely scenario if a licence deal does not materialize within the next 6-9 months. We now look forward to the outcome of Phase I with NEX-22 and more positive news flow from the partner projects.